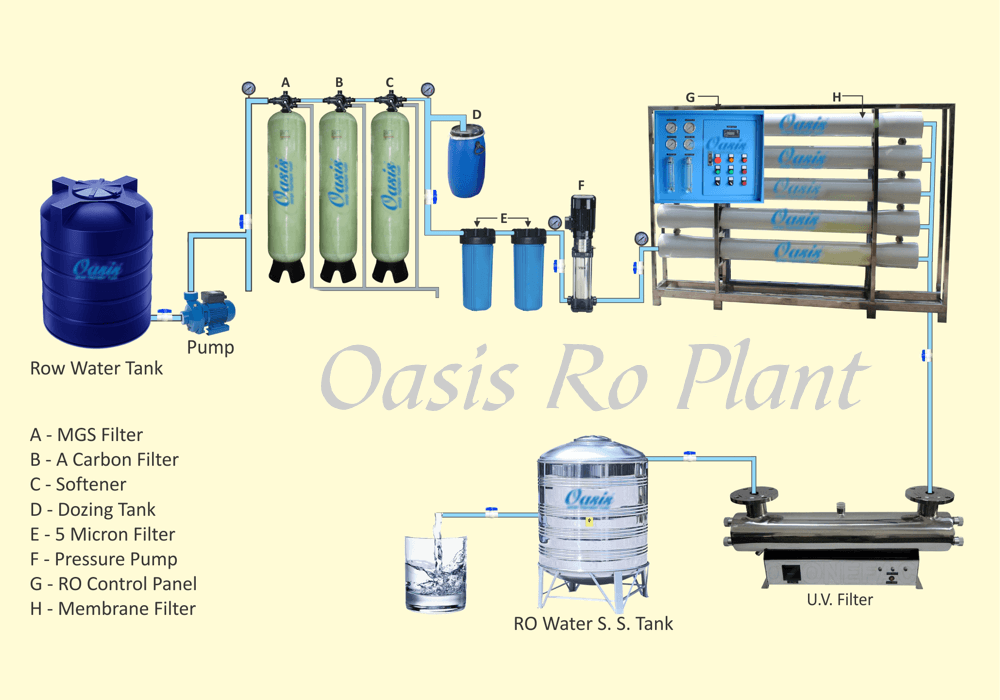
- Home Reverse osmosis (RO)
Reverse osmosis (RO)
Reverse osmosis removes contaminants from unfiltered water, or feed water, when pressure forces it through a semipermeable membrane. Water flows from the more concentrated side (more contaminants) of the RO membrane to the less concentrated side (fewer contaminants) to provide clean drinking water. The fresh water produced is called the permeate. The concentrated water left over is called the waste or brine.
A semipermeable membrane has small pores that block contaminants but allow water molecules to flow through. In osmosis, water becomes more concentrated as it passes through the membrane to obtain equilibrium on both sides. Reverse osmosis, however, blocks contaminants from entering the less concentrated side of the membrane. For example, when pressure is applied to a volume of saltwater during reverse osmosis, the salt is left behind and only clean water flows through.
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to remove ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property that is driven by chemical potential differences of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of dissolved and suspended chemical species as well as biological ones (principally bacteria) from water, and is used in both industrial processes and the production of potable water. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. To be "selective", this membrane should not allow large molecules or ions through the pores (holes), but should allow smaller components of the solution (such as solvent molecules, i.e., water, H2O) to pass freely.[1]
In the normal osmosis process, the solvent naturally moves from an area of low solute concentration (high water potential), through a membrane, to an area of high solute concentration (low water potential). The driving force for the movement of the solvent is the reduction in the Gibbs free energy of the system when the difference in solvent concentration on either side of a membrane is reduced, generating osmotic pressure due to the solvent moving into the more concentrated solution. Applying an external pressure to reverse the natural flow of pure solvent, thus, is reverse osmosis. The process is similar to other membrane technology applications.
Request an expert call back on Reverse Osmosis
Call us via +91 94311 14654 or visit us at Jokhi Ram Market, J.J Road, Upper Bazar, Ranchi, Jharkhand - 834001
Contact Us
We are always at your service. You can contact us at -
Jokhi Ram Market, J.J Road, Upper Bazar, Ranchi, Jharkhand - 834001
+91 94311 14654
[email protected]Quick Links
© Powered By CN Digital | Login